-
The origin of structural colors can date back centuries, spurred by early natural observations that intrigued scientists and sparked investigations into their underlying mechanisms1. Unlike conventional pigmented colors, structural colors emerge from the interaction of light with thin films or nanostructured materials, manifesting through phenomena like interference, diffraction, and scattering2–5. This light-matter interplay has attracted interest of researchers across a spectrum of disciplines, including physics, materials science, chemistry, and biology6–8. Their fundamental understanding has propelled a diverse array of applications across industries, from cosmetics and textiles to coatings and automotive finishes, offering a vivid and enduring alternative9. Additionally, their unique optical properties have spurred innovations in optical devices, sensors, displays, photonic materials, and inkless printing10–17. As research into structural colors progresses, their historical significance, fundamental principles, and versatile applications continue to inspire scientific inquiry and technological progress.
In recent years, different methods have been established for fabricating structural colors, such as electron beam lithography (EBL)18–22, focused ion beam milling (FIB)23,24, nano-imprinting25,26, and colloidal assembly27–31. The EBL and FIB milling techniques provide high precision and controllability over nanostructure patterns. However, these techniques are often time-consuming, expensive, and limited in scalability, rendering them less suitable for large-scale production32. Nano-imprinting, on the other hand, offers a cost-effective and scalable approach for replicating nanostructures over large areas. It allows for the production of structural colors with high throughput and relatively low cost compared to lithographic methods. However, nano-imprinting may suffer from issues such as pattern fidelity and mold durability33. Colloidal assembly techniques provide simplicity, versatility, and scalability for mass production. They enable the self-assembly of colloidal particles into ordered structures, facilitating the fabrication of structural colors with minimal equipment and cost. However, colloidal assembly may lack the precision and control achievable with lithographic methods, resulting in variations in color uniformity and optical properties34.
In addition to these above-mentioned methods, a recent advancement has surfaced: direct laser writing for the production of large-scale structural colors35–41. This technique harnesses laser technology to pattern nanostructures directly onto surfaces, presenting several distinct advantages over traditional techniques. Unlike lithography or milling, laser writing is a non-contact, one-step, maskless process, facilitating swift and precise fabrication of structural colors on a variety of substrates in ambient air environments42. Moreover, laser writing offers unparalleled flexibility, enabling rapid prototyping and on-the-fly modifications to designs. With its versatility and potential for scalable production, laser writing presents a promising new frontier for the fabrication of structural colors with applications across diverse fields such as optical filters, advanced displays, sensitive sensors, and photonic devices43–46.
Laser-writing structural colors can be in terms of on several mechanisms, including the generation of metallic nanoparticles through laser ablation47–50, the creation of nanogratings via laser-induced periodic surface structures51–55, and the formation of thin films by laser-induced surface chemical reaction such as oxidation and polymerization56–61. By leveraging laser technology, these methods enable precise manipulation of nanostructures on surfaces, facilitating the generation of vibrant structural colors. Metallic nanoparticles interact with light to produce vivid colors through localized surface plasmon resonances. Nanogratings create diffraction-based colors due to their periodic arrangement. Additionally, laser-induced oxidative layers can alter surface reflection, leading to color changes through interference and thus absorption. Coloring by laser-induced oxidative layers offer several advantages over other mechanisms35. Firstly, the oxidative layer approach typically results in higher mechanical stability compared to other methods relying on plasmonic nanoparticles or periodic nanogratings. Secondly, oxidative layer-based structural colors often exhibit a broader range of colors compared to other mechanisms. Finally, the manufacturing speed of laser oxidation can be faster compared to techniques involving the fabrication of complex nanostructures or the deposition of nanoparticles. The process of laser-induced oxidation can be relatively rapid and scalable, enabling efficient large-scale production without compromising color quality or stability.
In our recent work, we have successfully achieved scalable, wide-gamut, stable, and robust structural colors through laser-induced oxidation on TiAlN-TiN hybrid films62. While our previous studies have predominantly focused on investigating the influence of scanning speeds and laser powers, commonly referred to as dose, on the generated colors, the impact of laser pulse duration has remained unexplored. In the past decades, despite considerable attention being devoted to studying the effects of pulse duration on laser ablation processes63,64, its influence on the dynamics of laser oxidation, especially concerning structural color generation, has largely been overlooked. This research gap underscores the necessity for further exploration into the relationship between pulse duration and resulting structural colors, as well as its underlying mechanisms.
In this article, we further explore the laser-writing of structural colors on TiAlN-TiN bi-layered films, utilizing a range of pulse durations from 700 fs to 10 ps. Our investigation focuses on analyzing the concentration of injected oxygen element and the thickness of the oxide layer across different pulse durations. Furthermore, we investigate the relationship between the color difference and the oxidation process. We aim at providing a comprehensive investigation of how pulse duration affects the dynamics of laser oxidation. These insights are crucial for optimizing laser-writing techniques and achieving precise control over structural coloration processes.
-
Our experimental scheme is depicted in Fig. 1a−d. The laser coloring effect is applied to a bi-layered hybrid film consisting of a 60-nanometer-thick TiAlN layer, serving as the primary absorption layer, and a 50-nanometer-thick underlayer of TiN, acting as the reflective component. These two layers behave as a broadband absorber65 and are precisely deposited onto a 2-inch silicon wafer. Our TiAlN film exhibits a face-centered cubic NaCl-type phase as established in our preceding work with X-ray diffraction66, renowned for its high hardness, superior mechanical, and tribological properties, making it highly suitable for practical applications in robust structural colors. Following irradiation by a pulsed laser, an additional layer forms on the surface of TiAlN through laser-induced oxidation, as demonstrated in Fig. 1c. The interference of light reflection at the interfaces results in the generation of reflective structural colors, as illustrated in Fig. 1d. Essentially, the displayed colors can be adjusted by controlling the depth of laser-induced oxidation.
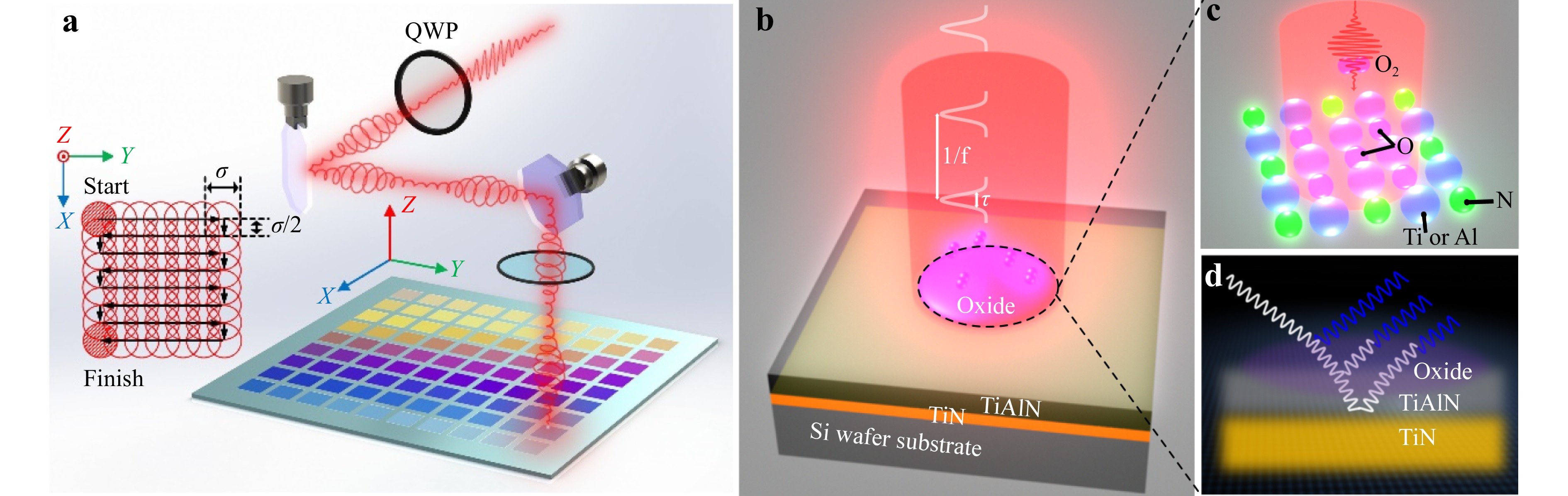
Fig. 1 Experimental setup a and scheme b of surface coloring by laser-induced surface oxidation on a TiAlN-TiN hybrid film. The employed pulsed laser operates at a repetition rate of f = 5 kHz, with a wavelength of 1030 nm, and a pulse duration (τ) ranging from 0.7 ps to 10 ps. Inset: schematic illustration of the meandering scanning. The black arrows indicate the scanning direction of laser beam. The distance between adjacent lines is σ/2, where σ is the laser focal spot diameter. c Laser-induced oxidation of TiAlN, which results in the interference of light reflection at different interfaces d, leading to the generation of structural colors that are dependent on the thickness of oxide layer.
Using a home-built laser marking machine, scalable colorful patterns are written on the TiAlN surface through meandering motion facilitated by two galvo mirrors. Through a FPGA motion controller, the spot reflected by the galvo mirrors is meandered along the planned path. The scanning scheme is shown in the insertion of Fig. 1a. The employed laser (Ultron Photonics) operates at a repetition rate of f = 5 kHz and pulse durations ranging from 0.7 ps to 10 ps. Original linearly polarized laser, upon traversing a quarter-wave plate, undergoes a transformation into circularly polarized laser, which is then utilized for irradiation. This conversion is essential because linear polarization often leads to the formation of laser-induced periodic surface structures, which can cause undesirable iridescence. Although perfect conservation of circular polarization is not achievable when it passes through Galvo mirrors, our experiments have consistently shown no evidence of formation of periodic structures. With a central wavelength of 1030 nm falling within the near-infrared spectrum, effective interaction with the TiAlN layer is ensured while minimizing thermal impact on the silicon substrate. Laser focusing was accomplished using a lens with a focal length of 15 cm, which effectively converged the incident beam, initially 3 mm in diameter, to a spot diameter of σ = 40 μm. The laser coloring experiments were performed in an ambient air environment. The laser pulse duration and energy are adjustable, providing flexibility to control the depth and characteristics of the laser-induced oxide layer on the TiAlN surface. This adjustability is crucial for fine-tuning the structural colors resulting from light interference at the material interfaces.
The structural colors exhibit high sensitivity to the surface oxide layer. Fig. 2a compares the simulated reflection spectra of two configurations: one with a transparent Al2O3 oxide layer atop a 30-nanometer TiAlN film coating on a 50-nanometer TiN substrate, and the other without Al2O3 oxide layer. It is observed that the presence of the oxide layer introduces an additional absorption peak in the ultraviolet spectral range, attributing to the interference of light reflection at the air- Al2O3 and Al2O3-TiAlN interfaces. Fig. 2b displays the simulated colors of TiAlN-TiN bi-layered films in CIE 1931 coordinates, shifting along a counter-clockwise direction as the TiAlN thickness reduces from 60 nm to 0 nm. Additionally, in Fig. 2c, upon oxidation of TiAlN, although the colors still change along the counter-clockwise direction with increased oxidation depth namely decreased TiAlN thickness, its gamut undergoes a noticeable shift compared to the pristine TiAlN-TiN film, transitioning from the yellow to blue region.
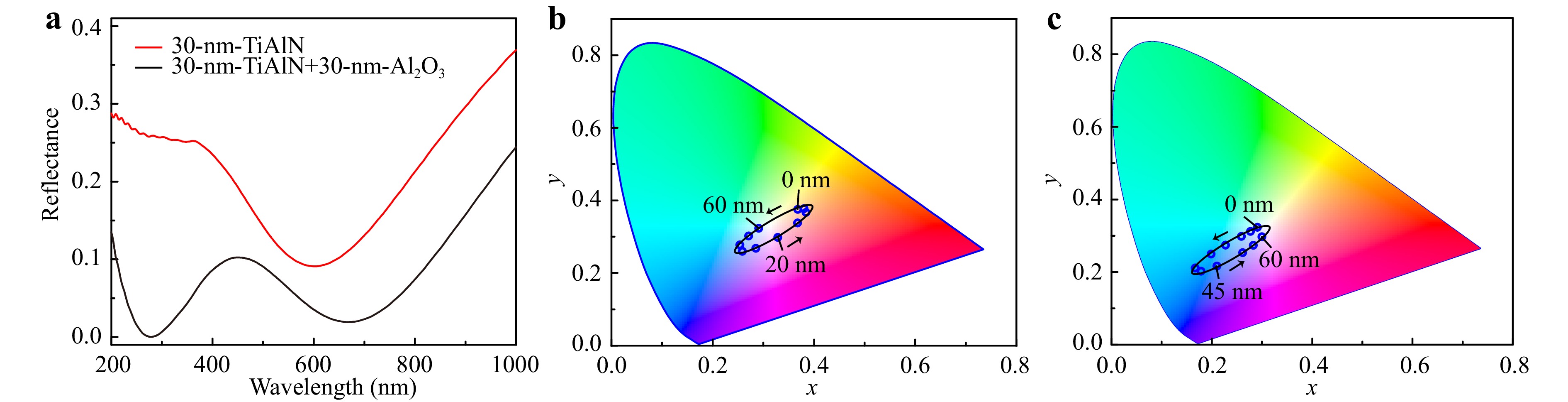
Fig. 2 a Simulated reflectance spectra at normal incidence of a 30-nm-thick TiAlN film on a 50-nm-thick TiN substrate (red curve), a stack of 30-nm- Al2O3 on 30-nm-TiAlN coating on a 50-nm-thick TiN substrate (black curve). b Numerically simulated CIE 1931 color coordinates for variable thickness of TiAlN decreasing from 60 nm to 0 nm along the arrows’ direction, and c for variable thickness of Al2O3 from 0 nm to 60 nm along the arrows’ direction. Please note that in c, the total thickness of TiAlN and Al2O3 keeps a constant at 60 nm. Therefore, the gradual increase of oxide layer depth corresponds to a reduction of the remained TiAlN thickness.
Fig. 3a-c depicts a series of matrix palettes generated through meandering laser scanning with a consistent line spacing of 20 μm. These palettes showcase a spectrum of structural colors, reflecting the interplay among laser power (P), scanning speed (v), and laser pulse duration (τ). The parameters of P and v determine the irradiated dose: $ D={4NP}/{{\text π} f{\sigma }^{2}} $, where N = f σ/v denotes the effective number of laser pulses delivered to the material that is inversely proportional to the scanning speed v, f and σ are the repetition rate and spot diameter of the laser, respectively. Therefore, to some extent, the parameters of P and v are potentially interchangeable in achieving specific laser treatment outcomes. For instance, a purple tone can be achieved either with low power and slow scan or with high power and fast scan settings. In Fig. 3a, at low laser powers and fast scanning speeds – low dose – the colors produced closely resemble those of the pristine surface. With the gradual increase of dose, a clear change of vivid colors is observed, transitioning from light blue through purple to yellow.
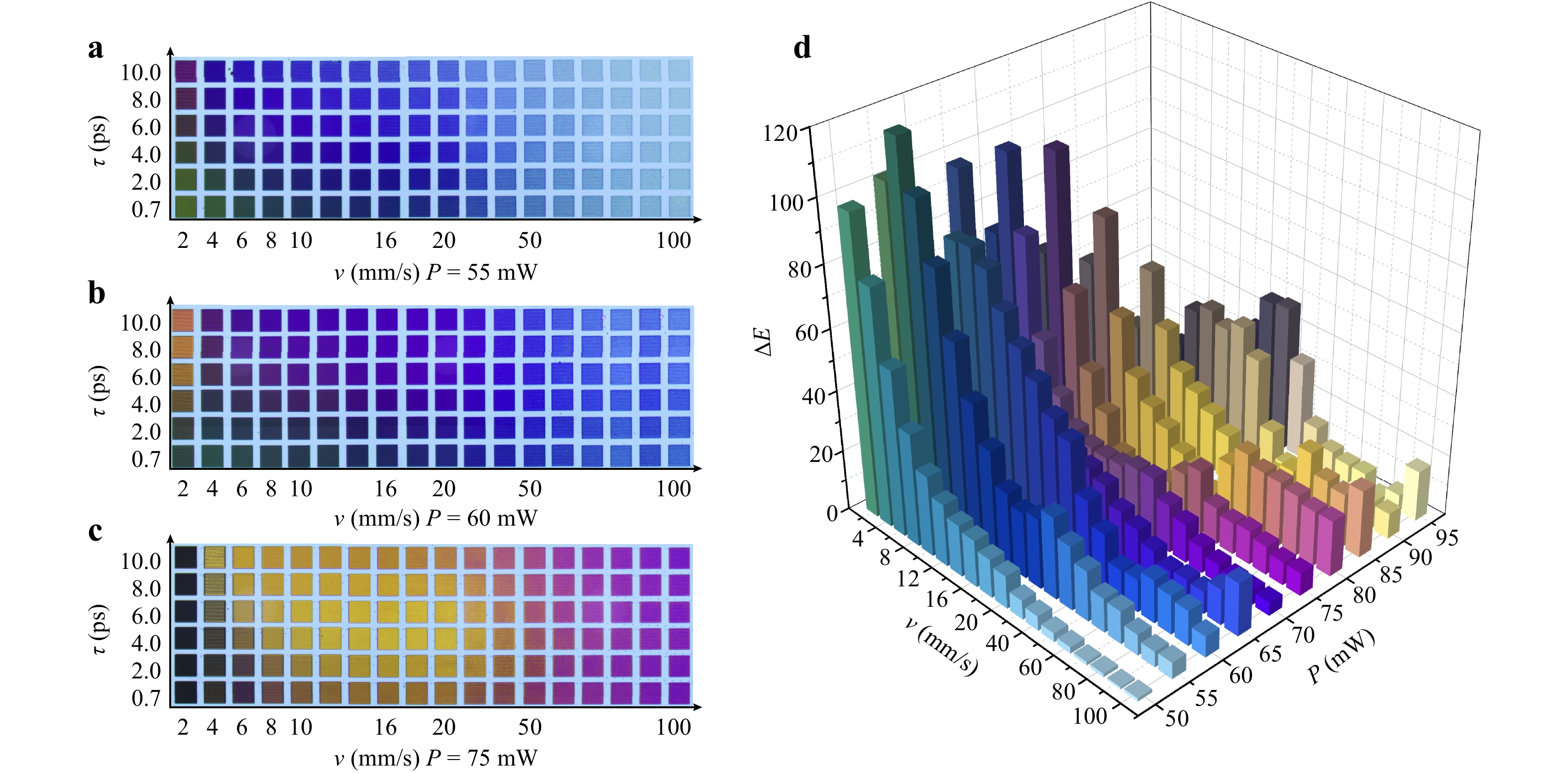
Fig. 3 a−c Matrix palettes that are generated through variable combinations of laser power, scan speeds, and pulse durations, revealing the diverse colors imparted to TiAlN surface as a result of the intricate interplay among these laser processing parameters. The size of each palette is 530 μm × 530 μm. d the color difference ($ \Delta {E}_{ab}^{*} $) between surfaces processed with two distinct pulse durations of 0.7 ps and 10 ps at identical laser power and scanning speeds. The color representations within the bars of d are true-to-life, extracted directly from the surfaces modified by the respective laser parameters, providing a visual reference of the actual colors achieved through laser treatment.
In contrast, the influence of pulse duration on the generated structural colors is not as pronounced as that of the irradiated dose. The variation in colors with respect to pulse duration is only noticeable at low laser powers and slow scanning speeds, as displayed in Fig. 3a, b. The difference in colors produced by laser pulses of 0.7 ps versus 10 ps, both at the same doses, is highlighted in Fig. 3d. At low powers, the color difference ($ \Delta {E}_{ab}^{*}={L}_{ab}^{10ps}-{L}_{ab}^{0.7ps} $) is highly sensitive to scanning speed, exhibiting a nonlinear increase as the scanning speed decreases. Conversely, at high laser powers, the $ \Delta {E}_{ab}^{*} $ become less predictable, especially at slow scanning speeds. This is attributed to the onset of material ablation, a process that alters the color generation mechanism, which is no longer solely due to laser-induced oxidation. The results in Fig. 3d further indicate that, indeed, the pronounced impact of pulse duration on coloring is most significant at low power and slow scanning speeds, in which regime the pulse-to-pulse accumulated laser-oxidation process is expected to be pre-dominated.
As depicted in Fig. 4, we further investigate how the laser-induced oxidation process influences surface colors by measuring the relative weight of oxygen element on the laser-treated surfaces with various parameters of P, v or N, and τ. To achieve this, we employ an analytical approach combining a scanning electron microscope (SEM) with an energy dispersive x-ray spectroscopy (EDX) detector. Additionally, we have configured the low-voltage electron beam in our EDX analysis to achieve shallow penetration depths, thereby enabling us to obtain a more precise assessment of the surface distribution on the TiAlN film. As shown by the insets in Fig. 4a, the two-dimensional EDX maps confirm that the TiAlN has been indeed oxidized after laser writing. First, we observe a linear increase in oxygen content as the laser power increases. Correspondingly, the colors gradually shift from light blue to yellow hues in a counter-clockwise direction, as depicted in Fig. 4b, which is in agreement with the numerically simulated results in Fig. 2. Under low-power irradiation, where the oxidation process is weak, the structural color remains close to the initial color of the pristine films, namely light blue. Conversely, under high-power illumination, where the oxidation process is strong, the upper layer of TiAlN becomes nearly transparent, causing the structural color to approach that of TiN, which is yellow. These findings highlight a strong correlation between laser-induced oxidation and the generated structural colors. The oxygen content also exhibits a linear increase with the number of irradiated pulses, as depicted in Fig. 4c. Similarly, the corresponding structural colors gradually vary from light blue to yellow in a counter-clockwise direction with the increasing degree of oxidation, as illustrated in Fig. 4d.

Fig. 4 Measured weight of oxygen content and corresponding CIE x-y chromaticity diagram at the surface of laser-processed areas as a function of laser power a, b effective number of irradiated pulses c, d and laser pulse duration e, f. Insets in a: two-dimensional EDX maps of nitrogen and oxygen of a laser-writing area. A portion of nitrogen has been replaced by oxygen, conforming the formation of oxide layer through laser irradiation.
However, upon investigating the impact of pulse duration, we discover that the relationship between laser-induced oxygen content and structural colors exhibits an opposite behavior compared to that of laser power and scanning speed. As shown in Fig. 4e, the weight of the oxygen element decreases with the increase of laser pulse duration, but the variation of color hues still follows a counter-clockwise direction, as depicted in Fig. 4f. These results suggest that the longer pulse results in a larger coloring change than the shorter pulse, despite the former exhibiting lower oxygen content.
-
To uncover the mechanisms behind this anomalous observation, we conduct laser-coloring experiments using single-pulse irradiation and analyze the height of the laser-treated surface by using atomic force microscopy (AFM). Fig. 5a presents bright-field optical microscopy images of laser coloring spots with different pulse durations. The colors vary from green to violet as the pulse duration increase, while the size of the laser-modified area remains independent of the pulse duration. This implies that the lateral heat diffusion is negligible during laser irradiation and the colors change with respect to pulse duration should arise from different thermal effect in the vertical direction. The corresponding dark-field optical microscopy images are depicted in Fig. 5b. In general, the relationship between surface roughness and brightness in dark field microscopy is attributed to the scattering of light from irregularities on the surface. Our observations indicate that longer pulse duration produce brighter spots under dark-field condition, suggesting an increase in surface roughness of the laser-modified areas with pulse duration. This increase in surface features and oxide distribution is further confirmed through high-resolution AFM, as illustrated in Fig. 5c-h, which provides topographical data with height information.
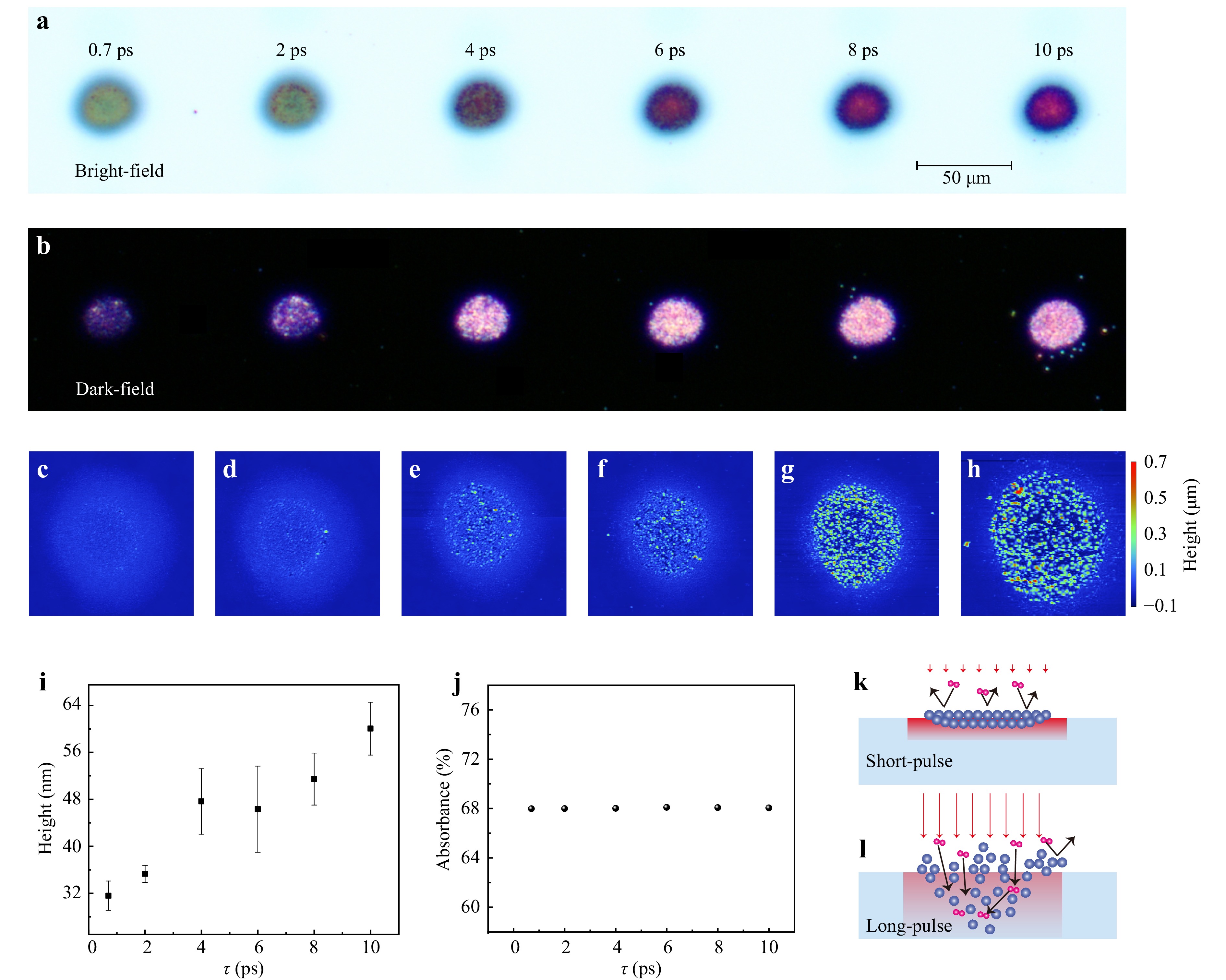
Fig. 5 Bright-field a and dark-field b optical microscope images, along with corresponding atomic force microscope images c-h, depicting the laser-illuminated surface after a single pulse with varying pulse durations. The applied laser power is 50 mW. i The average height of the laser-treated surface with different pulse durations. j Absorbance of the pristine TiAlN-TiN hybrid film with respect to pulse duration. k, l Schematic illustration of laser-induced oxidation by short- and long-pulse, respectively.
Next, we compare the average height of those AFM images in Fig. 5i. Despite the decrease in the weight of laser-generated oxygen content with the increase of pulse duration [e.g., Fig. 4e], the depth of the oxidized layer evidently increases with pulse duration. The increase of oxide depth with pulse duration helps to explain why the colors change along the counter-clockwise direction with the duration, which aligns with the numerical simulations depicted in Fig. 2c, because the structural colors transition along the counter-clockwise direction with the increase in depth of the oxide layer.
The oxidation of TiAlN under ultrafast pulsed laser irradiation may occur through two processes67. One channel involves photo-oxidation under a pulsed laser-excited nonequilibrium state, wherein the transient excitation of numerous hot electrons generating highly reactive species. Simultaneously, the dissociation of oxygen molecules under intense laser irradiation can interact with these reactive species and thus leading to the rapid formation of an oxidation layer before the material lattice heating up. The other process is the thermal-oxidation effect, in which materials are subjected to elevated temperatures, typically in the range of hundreds to thousands of degrees Celsius, leading to the gradual diffusion of oxygen molecules into the material and subsequent reacting with the TiAlN to form oxides. Therefore, it occurs after the heating of material via electron-phonon scattering and thus happens in a relatively longer time scale.
In the scenario of the nonequilibrium photo-oxidation mechanism, the oxidation degree is closely related to the transient electronic density and energy of the hot electrons. Due to its stronger peak intensity, the ultrashort femtosecond pulse tends to rapidly generate a dense oxidation layer on the TiAlN surface. This dense layer can prohibit the penetration of subsequent oxygen68 into the TiAlN film, as schematically illustrated in Fig. 5k. Therefore, the ultrashort pulse can produce a thin layer whereas is abundant of oxygen element. In the case of long-pulse irradiation, the oxidation process becomes gentler, resulting in a sparser oxide layer with higher oxidation depth but exhibiting lower oxygen content, as depicted in Fig. 5l.
In the case of thermal oxidation, both the depth of the oxide layer and the oxygen content are determined by the material’s temperature, which is influenced by the laser absorption coefficient and subsequent heat diffusion. As depicted in Fig. 5j, we confirm that the absorbance of the TiAlN-TiN hybrid film is independent of the pulse duration, indicating dominance of linear photon absorption. Consequently, the variation in oxidation degree can only arise from differences in the heat diffusion process. Specifically, longer pulse durations give rise to longer diffusion time and thus greater thermal diffusion depths, as illustrated in Fig. 5k, l Therefore, prolonged pulse irradiation results in lower temperatures, thereby generating deeper oxide layers while with weaker oxygen content.
-
In summary, we have demonstrated a comprehensive investigation into the influence of pulse duration on the laser coloring process of TiAlN-TiN hybrid films via ultrashort laser-induced surface oxidative reaction. The dynamics of laser-induced oxidation and resultant structural coloration in relation to pulse duration has been discussed, particularly under conditions of low laser power and slow scanning speeds. Our findings reveal that longer pulses induce more pronounced color changes due to increased oxide depth, despite its oxygen content is lower. Surface roughness and oxidation depth are analyzed using high-resolution atomic force microscopy to reveal the dependence of oxide depth versus pulse duration.
Moreover, our research elucidates the dual mechanisms of TiAlN oxidation under ultrafast pulsed laser irradiation, encompassing photo-oxidation in a non-equilibrium state and subsequent high-temperature oxidation following electron-phonon scattering. We highlight the ability of femtosecond pulses to rapidly form a dense oxidation layer, in contrast to longer pulses that facilitate deeper but less oxygen-rich oxidation profiles. These insights are crucial for strategically adjusting laser parameters to achieve desired structural color outcomes. The implications of our study extend to various domains within material science, with potential applications in advanced optical coatings, display technologies, and sensor systems that utilize the unique optical properties of structural colors. Precision control over structural colors through laser writing techniques paves a way for designing functional materials with customized optical responses. In conclusion, our findings not only in form the optimization of current laser-writing protocols but also inspire further exploration into the potential of laser-induced structural colors for innovative applications across diverse technological domains.
-
The TiN films were deposited on 2-inch single crystalline Si substrates via RF magnetron reactive sputtering at a temperature of 300 °C, with a power of 600 W, and nitrogen flow rate of 14 sccm, and argon flow rate of 56 sccm. The TiAlN films were deposited under the same parameters as TiN, with the power of the Al target set at DC 300 W. The film thicknesses were measured using a Stylus profiler. The permittivities of the deposited thin films were determined based on the measured thickness using a variable angle spectroscopic ellipsometer from Woollam. Scanning electron microscopy and energy dispersive X-ray spectroscopy analyses were conducted using a field-emission scanning electron microscope from Carl Zeiss, specifically the Gemini450 model. The depth of laser-induced oxide layer was measured by an atomic force microscope (Oxford Instruments Jupiter XR).
-
Numerical simulations of the reflection spectra of the hybrid films were conducted using the finite-difference time-domain (FDTD) method implemented in the Lumerical FDTD Solutions software package. A depolarized plane wave spanning from 200 nm to 1000 nm served as the light source for the simulation. Perfectly matched layers (PML) were applied to the top and bottom boundaries, while periodic boundary conditions were implemented in the x-y plane. A monitor was positioned 2000 nm above the source to capture reflection spectra. The permittivities of the materials were determined using the ellipsometer mentioned earlier.
-
The color characterization of the TiAlN surface was conducted using a computer vision script developed in Python. Laser-induced color blocks were identified in bright-field optical microscope images with a consistent brightness level. The average RGB value within a rectangular sampling area of 400 pixels×400 pixels was used to determine the color of each block. Subsequently, the standard RGB values were converted to CIE XYZ, CIE xy, and CIE Lab using the following formulas:
$$ forma{t_{{\text{RGB}}}}(c) = \left\{ {\begin{array}{*{20}{l}} {\dfrac{c}{{255}} \times 100,\begin{array}{*{20}{c}} {\begin{array}{*{20}{c}} {}&{} \end{array}{\text{if}}}&{c > 0.04045} \end{array}} \\ {{{\left(\dfrac{{c + 0.055}}{{1.055}}\right)}^{2.4}} \times 100,\;{\text{otherwise}}} \end{array}} \right. $$ (1) $$ \begin{split} {\text{X}} =& forma{t_{{\text{RGB}}}}({\text{R}}) \times 0.4124 + forma{t_{{\text{RGB}}}}({\text{G}}) \times 0.3576 + \\&forma{t_{{\text{RGB}}}}({\text{B}}) \times 0.1805 \\ {\text{Y}} =& forma{t_{{\text{RGB}}}}({\text{R}}) \times 0.2126 + forma{t_{{\text{RGB}}}}({\text{G}}) \times 0.7152 +\\& forma{t_{{\text{RGB}}}}({\text{B}}) \times 0.0722 \\ {\text{Z}} =& forma{t_{{\text{RGB}}}}({\text{R}}) \times 0.0193 + forma{t_{{\text{RGB}}}}({\text{G}}) \times 0.1192 +\\&forma{t_{{\text{RGB}}}}({\text{B}}) \times 0.9505 \\ \end{split} $$ (2) $$ \begin{gathered} {\text{x = }}\frac{{\text{X}}}{{{\text{X}} + {\text{Y}} + {\text{Z}}}}{\text{ }} \\ {\text{y = }}\frac{{\text{Y}}}{{{\text{X}} + {\text{Y}} + {\text{Z}}}} \\ \end{gathered} $$ (3) $$ k = \left\{ {\begin{array}{*{20}{l}} {{\text{X}}/95.047} \\ {{\text{Y}}/100.000} \\ {{\text{Z}}/108.883} \end{array}} \right. $$ (4) $$ forma{t_{{\text{XYZ}}}}(k) = \left\{ {\begin{array}{*{20}{l}} {{k^{1/3}},\begin{array}{*{20}{c}} {\begin{array}{*{20}{c}} {}&{} \end{array}{\text{if}}}&{k > 0.008856} \end{array}} \\ {(7.787 \times k) + (16/116),\;{\text{otherwise}}} \end{array}} \right. $$ (5) $$ \begin{gathered} {\text{L}} = forma{t_{{\text{XYZ}}}}({\text{Y}}) \times 116 - 16 \\ a = [forma{t_{{\text{XYZ}}}}({\text{X}}) - forma{t_{{\text{XYZ}}}}({\text{Y}})] \times 500 \\ b = [forma{t_{{\text{XYZ}}}}({\text{Y}}) - forma{t_{{\text{XYZ}}}}({\text{Z}})] \times 200 \\ \end{gathered} $$ (6) where c represents the single channel value of the standard RGB, respectively. The color difference (∆E*ab) was calculated as follows: $ \Delta {\text{E}}_{ab}^*{\text{ = }}\sqrt {{{({L_1} - {L_2})}^2} + {{({a_1} - {a_2})}^2} + {{({b_1} - {b_2})}^2}} $.
-
This work is supported by the National Natural Science Foundation of China (12474317 and 62105269). The authors thank the technical support from instrumentation and Service Center for Physical Sciences and from Instrumentation and Service Center for Molecular Sciences at Westlake University.
Ultrafast laser writing structural colors on TiAlN-TiN hybrid films
- Light: Advanced Manufacturing , Article number: (2025)
- Received: 18 May 2024
- Revised: 14 November 2024
- Accepted: 15 November 2024 Published online: 18 February 2024
doi: https://doi.org/10.37188/lam.2025.006
Abstract: We experimentally demonstrate ultrafast laser-writing wide-gamut structural colors on TiAlN thin film that is coated on TiN substrate via laser-induced surface oxidation. The experiments involve thorough control over laser parameters, including powers, scanning speeds and pulse durations, to investigate the interplay between these variables and the resulting structural colors. Surface characterization techniques, such as scanning electron microscopy, energy-dispersive x-ray spectroscopy and atomic force microscopy, are employed to analyze the properties of laser-induced oxide layers and their chromatic responses. Our findings indicate that while laser powers and scanning speeds are critical in determining the irradiated dose and the subsequent coloring effects, the pulse duration exerts a distinct influence, particularly at low laser powers as well as slow scanning speeds. Longer pulse durations are found to produce a more significant coloring change despite exhibiting lower oxygen content. This is attributed to the increased surface roughness and deeper oxidation layer achieved with prolonged pulses. We propose two oxidation mechanisms – photo-oxidation and thermal-oxidation – to elucidate the influence of pulse duration on laser coloring effects. These findings not only refine existing paradigms in laser-induced surface coloration but also stimulate further exploration of structural colors’ multifaceted applications across diverse technological contexts.
Research Summary
Ultrafast Laser Writing Structural Colors on TiAlN-TiN Hybrid Films
Liping Shi from Xidian university and colleagues have unlocked a method to inscribe vibrant structural colors on TiAlN-TiN hybrid films via ultrafast laser direct writing. By meticulously controlling laser power, scanning speed, and pulse duration, they trigger surface oxidation, generating a broad spectrum of vivid colors. They found that while laser powers and scanning speeds are critical in determining the irradiated dose and the subsequent coloring effects, the pulse duration exerts a distinct influence, particularly at low laser powers as well as slow scanning speeds. This breakthrough not only refines the understanding of laser-induced surface coloration but also opens new avenues for anti-counterfeiting devices and the creation of advanced optical coatings, potentially influencing industries from security to automotive.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.






 DownLoad:
DownLoad: