HTML
-
Luminescent physical sensors for monitoring temperature have shown great promise for use in the fields of aeronautical engineering, environmental engineering, and industrial processes1-4. They have distinguished advantages over traditional thermometers in terms of a fast response, a high sensitivity, and a tolerance to extreme atmospheres5-7. In particular, self-referencing optical thermometers do not require additional calibration of the emission intensity and are more accurate due to noninvasive operation8, 9.
Materials containing two different lanthanide ions are attractive for the construction of a self-referencing thermometer10. Lanthanide luminescent materials have sharp emission bands11, 12 and a large energy shift between the antenna absorption and lanthanide emission13, which distinguishes them from other luminescent materials, such as organic dyes or quantum dots14. In addition to these properties, lanthanide luminescence has a long lifetime, allowing time-gated techniques to increase the signal-to-noise ratio15. The temperature-dependent quenching and energy transfer between the dopant ions or between the host and lanthanide ion allow selective luminescent responses for different lanthanide ions with a change in temperature. Many lanthanide materials have been developed for temperature monitoring, such as lanthanide-doped inorganic nanocrystals16, metal-organic frameworks (MOFs)5, 17, polymers1, and co-doped complexes18-20. The reported maximum temperature sensitivities of recent systems involving energy transfer between two different lanthanide ions for ratiometric emission measurements are collected in Table S13. The stoichiometry of these systems is a critical parameter, and the sensitivities usually vary widely with temperature.
However, it is difficult to obtain exactly the same concentrations and qualities from different prepared batches of doped materials; the distance between the donor ion and acceptor ion, which is a crucial parameter for energy transfer, is random and can only be estimated by statistical treatment, and a mixed system with these materials allows donor-donor and acceptor-acceptor energy migration, making the kinetics of transfer more complicated. Hence, the hetero-dinuclear lanthanide complex, with its fixed distance between donor and acceptor12, its definite structure and stoichiometric ratio of donor and acceptor, has great potential for development as a luminescent self-referencing thermosensitive indicator. The donor and acceptor in the dinuclear complex are fixed at a certain distance so that the pairwise energy transfer rates can be obtained without statistical treatment. With a stoichiometric arrangement of different lanthanide ions at each binding center, the intramolecular energy transfer between the ions is scarcely interfered with by donor-donor and acceptor-acceptor energy migration. The antenna effect of the chelating chromophore, which possesses a large absorption cross section, allows more incident light to be absorbed and transferred to a lanthanide ion, making such complexes brighter than most lanthanide-doped inorganic nanocrystals. Unsurprisingly, few literature reports18, 21 include an investigation of the energy transfer processes using lanthanide hetero-dinuclear complexes - and even then with the co-presence of homo-dinuclear species in the complex mixture. Even though great effort has been expended to synthesize pure hetero-polynuclear complexes22, 23 with different chemically similar lanthanide ions, there remains a considerable need for a simple strategy for controlling the formation of hetero-dinuclear complexes. This is a severe challenge for lanthanide chemists.
Herein, we report a pair of hetero-dinuclear lanthanide complexes, cycTb-phEu and cycEu-phTb, as molecular-based luminescent temperature sensors. In order to avoid mixing the two chemically similar lanthanide ions, europium and terbium, they are situated in two distinct binding sites using two different synthesis steps. The clear validation of the metal ratio and the fixed distance between the energy donor and acceptor make the complexes outweigh other materials, such as doped crystals, MOFs and polymers, both in terms of the understanding energy transfer and in temperature sensing performance. The 1, 10-phenanthroline (phen) moiety serves as the chromophore to sensitize europium and terbium, giving an increased temperature-dependent luminescence emission ratio for europium over terbium. The luminescence from terbium and from europium is reduced in intensity with different rates as the temperature increases, and it involves the process of energy transfer from the chromophore to each ion and that from terbium to europium. Complexes cycTb-phEu and cycEu-phTb (Fig. 1) exhibit remarkably different photophysics due to the uniqueness of the two metal binding centers, and their energy transfer processes have been studied in detail.
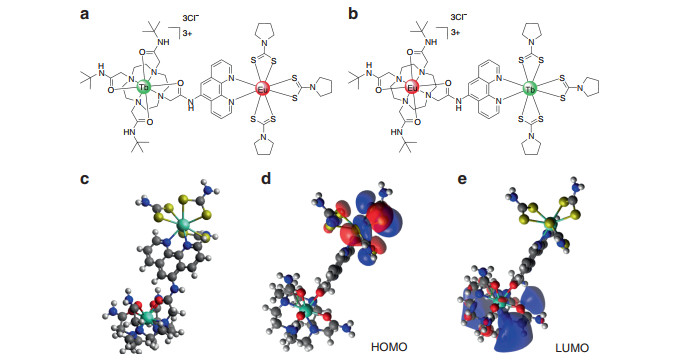
Fig. 1 The structures of complexes.
a cycTb-phEu and b cycEu-phTb, and c optimized geometry of the simplified 100-atom structure of cycTb-phEu as determined using the PBeh-3c functional24 with basis def2-SVP25 and effective core potentials for Tb and Eu26 in ORCA27 (refer to the SI for details). The charge-transfer nature of the d HOMO and e LUMO are shown
-
Refer to Fig. S1 for the structures and names of the complexes. The 166-atom structure of cycTb-phEu was modeled by MOPAC28, 29 using the semi-empirical RM1 software in the LUMPAC program30, 31, and the structure is shown in Fig. S13. Both Tb and Eu are 8-coordinated. Eu is coordinated to six S and to two N, whereas Tb is coordinated to four O and four N. A simplified 100-atom structure with aliphatic rings replaced by CH2 groups was employed for the optimization using the PBeh-3c functional in ORCA24-27 (Fig. 1). A comparison of the bond distances for these two different optimizations is provided in Table S10.
-
Scheme S1 shows the synthesis of the ligands and complexes. Ligand 2 (cyclen 1, 10-phenanthroline) was prepared by a substitution reaction of 2-bromoacetyl bromide with 1, 10-phenanthrolin-5-amine in the presence of K2CO3 in DCM for 22 h to give 1, which was followed by coupling with a triarmed cyclen in MeCN in the presence of NaHCO3 at room temperature, giving 2 in a 95.8% yield. The triarmed cyclen 1 was prepared via two-step substitution reactions according to the literature13. Complexes 3 (cycLn1-phen) were formed by coordinating lanthanide chlorides with one equivalent of ligand 2 in a mixed solution of MeOH and H2O at room temperature, followed by precipitation with diethyl ether from methanol. Taking advantage of fast coordination to Ln(Ⅲ) ions and less interference with the energy transfer from the 1, 10-phenanthroline chromophore to the Ln(Ⅲ) ions, pyrrolidine dithiocarbamate was chosen as the ligand for coordination with the second lanthanide ion. The synthesis of complexes 4 (cycLn1-phLn2) was achieved by coordinating lanthanide chloride with one equivalent of complex 3 and three equivalents of ammonium pyrrolidine-1-carbodithioate at room temperature, followed by precipitation with diethyl ether from methanol. The proton nuclear magnetic resonance (1H NMR) spectra of the La motif structure showed that three pyrrolidine dithiocarbamates were coordinated to the complex, which is consistent with a previous report32. This synthetic strategy provides a platform to obtain hetero-dinuclear complexes that consist of chemically similar lanthanide ions, one of which is not present in both ligand structures.
-
The singlet and triplet energy levels were experimentally studied by using the La motif complex, cycLa-phLa, since La3+ does not exhibit 4f-4f electron transitions. The large La3+ ion generates a heavy atom effect towards the surrounding organic ligand and induces spin-orbit coupling, which accommodates intersystem crossing from singlets to triplets. Thus, phosphorescence occurs. The fluorescence from the solid La3+ complex was measured at room temperature, while the phosphorescence was recorded at 77 K (Fig. 2). The room temperature emission under broad band 273 nm excitation was observed for cycLa-phLa with a maximum at 398 nm (25121 cm−1) (Fig. 2b), which is attributed to a π-π* singlet transition of the ligand. At lower temperature, the fluorescence intensity decreases, the band redshifts, and phosphorescence from T1 dominates at longer wavelengths (Fig. 2a). The zero phonon line of the T1 → S0 transition is at 497 nm (20, 124 cm−1), and there is a progression of the totally symmetric ring C = N mode at 1471 ± 6 cm−1 to lower energy (compare with the Fourier transform infrared (FT-IR) spectrum, Fig. S2). The excitation spectrum of the phosphorescence (Fig. S3a) demonstrates the population of T1 from a singlet state at ~400 nm. The excitation spectrum of the singlet fluorescence (Fig. S3b) shows a further singlet state at 273 nm. A strong band at or near this wavelength is observed for all of the cycLn-phLn systems in solution, for example, cycLa-phEu (Fig. 2c). In fact, the room temperature excitation spectra of cycEu-phLa (Fig. S4) and cycLa-phEu (Fig. S5) demonstrate the participation of other singlet states in the energy transfer routes from the ligand to Eu3+. Notably, by comparing the intensity of the ligand and Eu3+ absorption bands in the excitation spectra, the ligand-metal energy transfer is more efficient when Eu3+ is bound to the phen rather than to the cyclen moiety (Figs. S4 and S5).
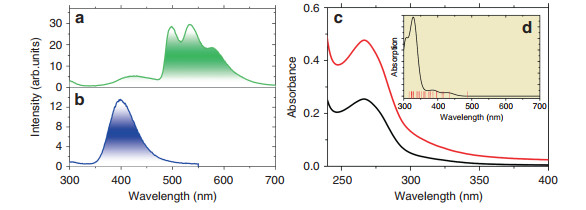
Fig. 2 Optical spectra.
The 77 K phosphorescence a and 298 K fluorescence b of cycLa-phLa (λexc = 273 nm) in the solid state. c Room temperature absorption spectrum of cycLa-phEu in buffer solution at pH 7.4 at two concentrations: black, 5 μM; red, 10 μM. The insert d shows the simulated absorption spectrum in the gas phase between 300 and 700 nm as determined from LUMPAC30 with the triplet levels calculated to low energy of 316 nm marked by vertical lines. The singlet transitions were broadened with widths of 2000 cm−1The triplet lifetime was determined to be 0.26 s at 77 K (Fig. S6a), whereas the singlet lifetime was measured at room temperature to be 1.6 ns (Fig. S6b).
-
The emission spectra of complexes cycTb-phEu and cycEu-phTb were measured in the solid state at 10 K with excitation at 355 nm into the ligand absorption band (Fig. 3). These emission spectra exhibit the characteristic Eu3+ and Tb3+ emission bands: 5D0 → 7FJ (J = 0-6) for Eu3+ and 5D4 → 7FJ (J = 6-4) for Tb3+. In each case, the emission from Eu3+ dominates with the forced electric dipole transitions 5D0 → 7FJ (J = 2, 4) being the strongest and more intense than the magnetic dipole-allowed transition 5D0 → 7F1. The 5D0 → 7F0 transition, which is active for Eu3+ situated at sites with Cn, Cnv, and Cs symmetry, was observed as a single sharp band in each spectrum, indicating that only one type of complex is present. The spectral bands are listed in Tables S1 and S2 together with the derived Eu3+ and Tb3+ energy level data. For Eu3+, the major difference between the two spectra is the stronger relative intensity and number of resolved bands of the 5D0 → 7F4 transition for cycEu-phTb. Interestingly, the emission bands from either Eu3+ or Tb3+ situated in the more rigid cyclen binding site are sharper than when Eu3+ or Tb3+ is coordinated in the phenLn(pdtc)3-binding site in both dinuclear complexes.
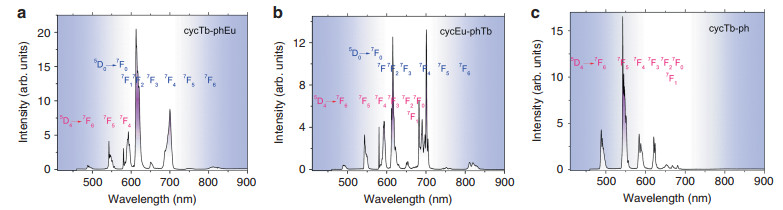
Fig. 3 The emission spectra (λexc = 355 nm) of complexes.
cycTb-phEu a, cycEu-phTb b, and cycTb-phen c at 10 K in the solid state. The Tb3+ transitions are marked in red, and those of Eu3+ are marked in blue. Refer to Tables S1 and S2. The relative intensities are not to scale for a-cThe hypersensitive transition 5D0 → 7F2 is more influenced by the coordinative environment than other forced ED transitions and serves as an indicator of the local symmetry of a coordination site33. The area ratio of the 5D0 → 7F2 transition to that of 5D0 → 7F1 is 3.93 for cycTb-phEu and 2.33 for cycEu-phTb. The forced electric dipole transition, 5D0 → 7F6, exhibits a weak emission intensity in the luminescence spectrum of each binuclear complex.
The 10K emission spectrum of cycTb-phen is displayed in Fig. 3c. The near-environment of Tb3+ is the same as in cycTb-phEu (Fig. 3a); however, the (pdtc)3Eu moiety is absent. The Tb3+-Eu3+ distance in cycTb-phEu is 10.6 ± 0.1 Å. The crystal field levels of Tb3+, as deduced in Table S1 from the Tb3+ emission spectra in Fig. 3a, c, are the same within experimental error for cycTb-phen and cycTb-phEu. For example, the splittings of the J-multiplets 7F6 and 7F5 are 407 ± 5 cm−1 and 494 cm−1, respectively, in each case. This gives an indication of the "spectroscopic vision" of Tb3+ and shows the atomic core-like nature of 4f orbitals so that the crystal field experienced by Tb3+ is effectively the same for both systems.
-
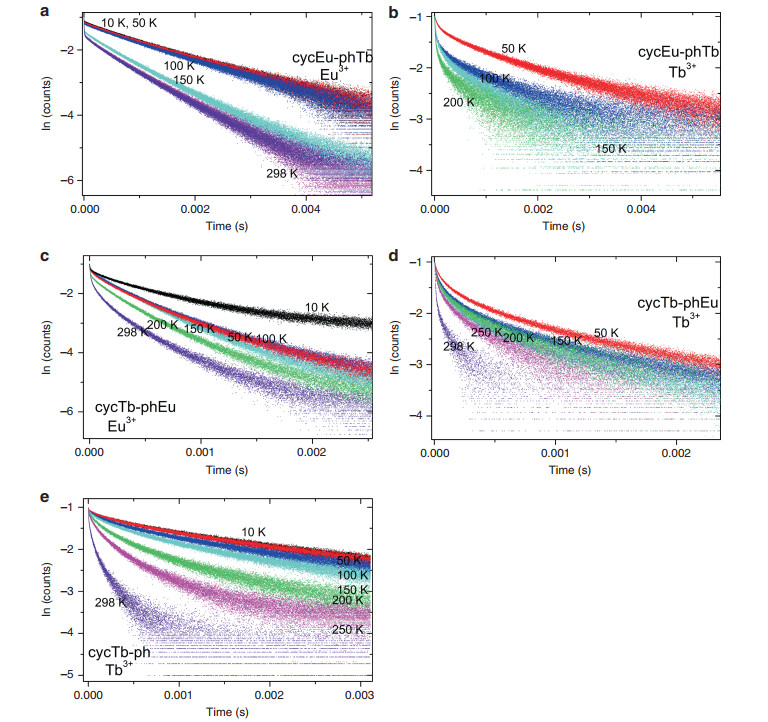
The intensity of emission was monitored as a function of time after pulsed excitation for the 5D0 (Eu3+) and 5D4 (Tb3+) states by measuring the emission decays for the 5D0 → 7F4 transition of Eu3+ and for the 5D4 → 7F5 transition of Tb3+ because the spectral bands of these transitions are well-separated from those of other transitions. The excitation wavelength, 355 nm, overwhelmingly excites the phen antenna, which may then transfer energy to Eu3+ and Tb3+. A further energy transfer from Tb3+ to Eu3+ is well-documented in the literature; 34-40 however, the use of excitation at 490 nm produced very weak emission in the present study due to weak absorption. Thus, we were unable to directly study this energy transfer process using this excitation wavelength. Fig. 4a-d display the Eu3+ 5D0 and Tb3+ 5D4 emission decays for cycTb-phEu and cycEu-phTb at various temperatures, whereas Fig. 4e shows the Tb3+ decay for cycTb-phen. An analysis of the decay curves is presented in Tables S3-S7 using mono- and bi-exponential functions and direct integration of the decay curves. Generally, the decays are not mono-exponential due to the initial faster decay, and the approach to mono-exponential behavior increases with time, as exemplified in the fits to the decay curves after ~0.2 ms. The Eu3+ decay in cycEu-phTb, as shown in Fig. 4a, comes closest to the behavior of mono-exponential decay. Taking the values measured after ~0.2 ms following the decay pulse, the lifetimes of the Eu3+ 5D0 excited state in complexes cycTb-phEu and cycEu-phTb decrease slightly from 0.34 to 0.25 ms and from 0.64 to 0.58 ms, respectively, as the temperature increases from 10 to 298 K due to multiphonon relaxation and back-transfer to 5D1. The Tb3+ 5D4 lifetimes exhibit greater changes: in cycTb-phEu, from 0.42 to 0.11 ms (from 10 K to 298 K) and in cycEu-phTb, from 0.65 to 0.13 ms (from 10 K to 200 K). The changes follow the same patterns as above but are different in magnitude upon considering the values of the "steady-state" lifetime, τST (Tables S4 and S7). Additional processes besides multiphonon relaxation are thus involved, such as energy transfer from Tb3+ to Eu3+.
-
There have been many previous literature studies that have investigated this energy transfer with varying concentrations of Tb3+ and Eu3+ (Table S9). Ideally, this energy transfer can be assessed from a comparison of the luminescence decays of cycTb-phEu and cycTb-phLa; however, since we did not synthesize the latter complex, we considered the decays of cycTb-phEu and cycTb-phen as an alternative estimation. The likely energy transfer pathways34-40 are from Tb3+ 5D4 (20, 482 cm−1) to Eu3+ 5D1 (with the lowest crystal field level at 19, 048 cm−1 as shown in Fig. S5 (literature value at ~19, 100 cm−141) and to Eu3+ 5D0 (17, 233 cm−1, Table S1), with energy differences of 1434 and 3249 cm−1, respectively. The participation of 5D1 in Fig. 3a and b is not evident, particularly when compared with the spectrum of cycLa-phEu (Fig. S5), supporting the direct energy transfer from Tb3+ to Eu3+ 5D0 rather than to 5D1. From first order selection rules42, the pathways of 5D4-7F6, 7F5 involve an electric quadrupole and/or forced electric dipole nonradiative transition. The transition 7F0-5D0 is dipole and quadrupole forbidden to first order, although the thermally assisted 7F1-5D0 transition is both forced electric dipole and electric quadrupole allowed. Both 5D4-7F6 and 7F0-5D0 are forbidden by the exchange43 selection rule, |J - J'| = 0, 1 with J = 0 ↔ J' = 0 forbidden. The transfer via the charge-transfer state Eu2+-Tb4+ was not considered.
The long-term (> 0.2 ms) luminescence decay constants (i.e., reciprocal lifetimes) of cycTb-phEu (kTbEu) and cycTb-phen (kTb) are listed in Table 1 together with the difference in kTbEu-kTb = kET', taken to indicate the Tb3+ → Eu3+ energy transfer rate. Alternative descriptions of the energy transfer rate by employing kET and kET" are also defined in the table. The magnitude of kTb does not change markedly with temperature (by 10% from 10 K to room temperature). In contrast, kTbEu considerably increases by 270% with temperature, and kET' can be described by an exponential growth model (Fig. S8), clearly indicating the importance of temperature in the energy transfer process, which is understood as the thermal population and involvement of 7F1. Considering only the Eu3+ 7F0 and 7F1 J-multiplets, with the mean energy of the latter equal to 369 cm−1 (from Table S1), and following the description in ref. 44, the energy transfer rate can be expressed as:
$$ k_{{\rm{ET}}}\left( {{\rm{Tb}} \to {\rm{Eu}}} \right) = aN\left( {\,{}^7{\rm{F}}_1} \right){\rm{x}}T + bN\left( {\,{}^7{\rm{F}}_0} \right) $$ (1) $$ k_{{\rm{ET}}}\left( {{\rm{Tb}} \to {\rm{Eu}}} \right)/N\left( {\,{}^7{\rm{F}}_0} \right) = a \times 3{\rm{exp}}\left( { - 369/kT} \right){\rm{x}}T + b $$ (2) Temp (K) Rate constant (s−1) kTbEu kTb kET' kET kET" kr 10 2398 1555 843 943 35, 507 32, 062 50 2421 1545 876 1103 29, 165 27, 863 100 2740 1523 1217 1493 34, 131 49, 826 150 3086 1583 1503 2118 51, 931 64, 851 200 3751 1563 2188 3494 117, 128 84, 531 250 5618 1687 3931 5858 132, 653 89, 047 298 8811 1714 7096 21678 159, 544 123, 854 Temperature variation of Tb-Eu energy transfer rates deduced from the long-term lifetimes (kTbEu-kTb = kET'), direct subtraction of decay curves (1/τ1 = kET, with τ1 from Table S8), and steady-state lifetimes (1/τST(cycTb-phEu)-1/τST(cycTb-phen) = kET", with τST from Tables S4 and S7). The steady-state lifetime is determined by integration of the decay curves: $\tau _{{{ST}}} = \mathop {\int }\limits_0^\infty \frac{{I(t)}}{{I(0)}}dt,$ refer to the discussion above Table S3. The fitted parameter kr = 1/τ2 is from Table S7 Table 1. Temperature variation of long-term decay constants, kTbEu and kTb, for 5D4 Tb3+ emission in cycTb-phEu and cycTb-phen
where a and b are constants; the partition function is given by [1 + 3exp(-369/kT)] = P, where k is the Boltzmann constant; the average 7F1 energy is 369 cm−1; and the populations of 7F1 and 7F0 are N(7F1) = n1 = 3exp(-369/kT)/P and N(7F0) = n0 = 1/P, respectively. Taking the results from Table 1, Fig. 5a shows a fit of the kET' values from 10 to 298 K with the use of Eq. 2.
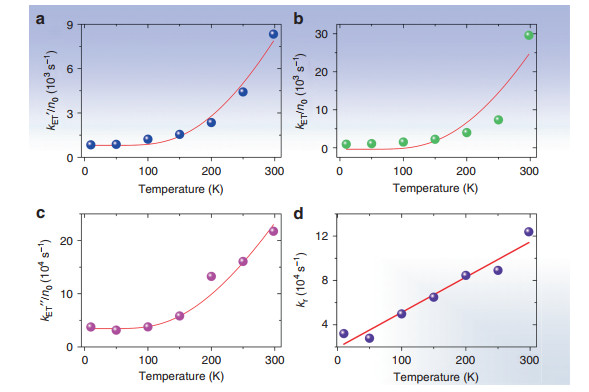
Fig. 5 Simulation of experimental values for the Tb3+ - Eu3+ energy transfer rate vs. temperature using Eq. 2.
(a) kET' with values of a = 46.9 K−1s−1 and b = 809 s−1; b kET with values of a = 166 K−1s−1 and b = -369 s−1. c kET" with values of a = 2793 K−1s−1 and b = 34500 s−1. d Linear plot of rise time of subtracted decay curves (Table 1) against temperature with a fitting of y = (1.93 ± 0.58) + (318 ± 32)x, Radj2 = 0.941The energy transfer pathway at low temperature, therefore, involves 7F0 and phonon(s) emission:
$$ {{}^{5}{\rm{D}}_{4}{\rm{Tb}}^{3 +}\left( {20482} \right) + \, {}^{7}{\rm{F}}_{0}{\rm{Eu}}^{3 + }\left( 0 \right) \to \,{}^{7}{\rm{F}}_{5}{\rm{Tb}}^{3 + }\left( {2019 - 2513} \right)} \\ {+ \, {\rm{Eu}}^{3 + }\, {}^{5}{\rm{D}}_{0}\left( {17233} \right) + {\rm{phonon}}\left( {736 - 1230}\right)} $$ and/or
$$ {{}^{5}{\rm{D}}_{4}{\rm{Tb}}^{3 + }\left( 20482 \right) + {}^{7}{\rm{F}}_{0}{\rm{Eu}}^{3 + }( 0 ) \to {}^{7}{\rm{F}}_{6}{\rm{Tb}}^{3 + }\left( 0 - 405 \right)} \\ {+ \, {\rm{Eu}}^{3 + }\, {}^{5}{\rm{D}}_{1}\left( 19048 \right) + {\rm{phonon}}\left( 1029 - 1434 \right)} $$ where representative ranges of energies (in cm−1) are given in parentheses; or it involves the participation of 7F4 Tb3+ with the absorption of a low energy phonon. The energy transfer rate is higher at room temperature because the forced electric dipole transition, 7F1 → 5D0, is involved:
$$ {}^{5}{\rm{D}}_{4}{\rm{Tb}}^{3 + }\left( {20482} \right) + {}^{7}{\rm{F}}_{1}{\rm{Eu}}^{3 + }\left( {300 - 442} \right) \to {}^{7}{\rm{F}}_{4}{\rm{Tb}}^{3 + }\\ \left( {3289 - 3908} \right) + {\rm{Eu}}^{3 + }\, {}^{5}{\rm{D}}_{0}\left( {17233} \right) \pm {\rm{phonon}} $$ Alternatively, the decay curves of cycTb-phEu and cycTb-phen were directly subtracted (Fig. S9) and the resulting plots were well-fitted by a bi-exponential function (Table S8). The lifetime τ1 represents the reciprocal of the energy transfer rate from Tb3+ → Eu3+, kET (listed in Table 1). The values are comparable with those for kET', except that the room temperature value is much higher. Figure 5b shows a plot of the experimental values of kET against inverse temperature and displays a fit using Eq. 2 with a similar result as in Fig. 5a. The fits of the subtracted curves (Fig. S9) produce a rise time, τ2, in each case, which is equal to the reciprocal of the rate constant, kr. This rate constant gives a linear plot against temperature, as shown in Fig. 5d, which is expected for the phonon occupation number of a one phonon process. Finally, the plot included in Fig. 5c uses another alternative description of the energy transfer rate, kET", which was derived from steady-state decay lifetimes. Although the numerical values differ, the three fittings serve to confirm the importance of the participation of 7F1 in the energy transfer process at room temperature.
The energy transfer efficiency from Tb3+ → Eu3+ (ƞET = 1- kTb/kTbEu) has very different values when calculated using the three alternative sets of rate constants. The value is above 0.9 and is reasonably independent of temperature when using steady-state lifetimes.
-
The ligand triplet state energy of cycLa-phLa was experimentally determined to be 20, 124 cm−1 (Fig. 2), which is below the 5D4 energy (20, 482 cm−1) of Tb3+ in cycTb-phEu. Nevertheless, strong emission is observed from cycTb-phen at room temperature upon excitation into the ligand singlet state to populate the 5D4 state (Fig. S7). The 5D0 level (17, 233 cm−1) of Eu3+ lies sufficiently below the triplet state (~3300 cm−1) to avoid back-transfer. In addition to triplet T1 - metal transfer, there are also opportunities for transfer to the lanthanide ions from higher ligand levels upon 355 nm excitation.
The calculation of singlet and triplet levels of cycTb-phEu was performed by first optimizing the structure using the Sparkle/RM1 method in MOPAC28. Then, the optimized geometry was used in LUMPAC with standard settings to calculate the excited states using Zerner's intermediate neglect of differential overlap (ZINDO/S) semi-empirical method30. Very different triplet state energies resulted when the number of states in the calculation was varied. Figure 2d displays the result with 25 states, where the lowest triplet state energy was calculated at 20, 501 cm−1 (487.8 nm). In this calculation, there are 25 triplet states and 19 singlet states with transition wavelengths to S0 that are longer than 315 nm, with the lowest energy singlets calculated at 391, 416, and 433 nm. The schematic relative energies of the ligand Tb3+ and Eu3+ levels in cycTb-phEu are shown in Fig. 6 with ligand levels from the above calculation and with the metal ion levels determined experimentally or from other systems. The ability to assign definitive energy transfer pathways is therefore complex.
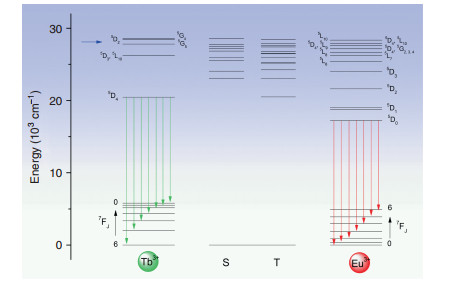
Fig. 6 Simplified energy level diagram for Tb3+-Eu3+ dyads of singlets (S) and triplets (T) of ligand, and lower levels of Tb3+ and Eu3+ ions.
The J-multiplet energy levels given for Tb3+ and Eu3+ may not be exact (and are split by the crystal field) but are illustrative of relative energies. The green and red vertical arrows mark emission from Tb3+ and Eu3+, respectively. The blue horizontal arrow denotes the 355 nm excitation energyThe 355 nm excitation into the dyad system can populate the ligand level at ~360 nm (Fig. S4), which we associate with the calculated level (Fig. 6) at 28, 550 cm−1. The gap below this singlet level is spanned by 1 phonon (~770 cm−1) so that internal conversion can be fast. In fact, all of the singlet-singlet gaps down to the lowest singlet state calculated at 23, 084 cm−1 (433 nm) are spanned by one phonon. The latter ligand singlet level is identified with the weak band at 437 nm in Fig. S4. From this figure, it is demonstrated that the rate of internal conversion is slower than the rate of antenna-metal ion energy transfer for these two singlet states. The energy transfer rates from not only these two states, but all of those potentially populated by 355 nm excitation, to the Eu3+ levels were investigated using the LUMPAC program, and the results from three different optimizations and calculations are displayed in Tables S11 and S12. The calculated energies represent those from vertical Franck-Condon transitions to unrelaxed excited states. The LUMPAC program neglects intra-ligand intersystem crossing and internal conversion processes. However, it represents the most informative analysis of antenna-metal energy transfer processes currently available. It is evident from Tables S11 and S12 that the major energy transfer pathways from the ligand to Eu3+ involve upper Eu3+ J-multiplets, followed by internal nonradiative relaxation to 5D0.
-
To investigate their potential application for luminescence thermometry, the temperature-dependent photophysical properties of complexes cycTb-phEu and cycEu-phTb were examined in terms of both the luminescence intensity and lifetime, at intervals between 10 and 298 K (Fig. 7). Three selected temperatures for the emission spectra of these compounds are employed in Figs. S10a, b to show the trends in emission intensity. Moreover, the changes in intensity of the Tb3+ 5D4 → 7F5 and Eu3+ 5D0 → 7F4 transitions clearly demonstrate the trends. For cycTb-phEu, the major decrease in intensity occurs above ~50 K for Tb3+, and it is above ~100 K for Eu3+, as shown in Fig. S11a, whereas the decreases begin to occur at lower temperatures for cycEu-phTb (Fig. S11b). The changes in the long-term lifetimes of the 5D4 and 7F0 states for these complexes are shown in Fig. 7c, d. These changes are greater for Tb3+ than for Eu3+.
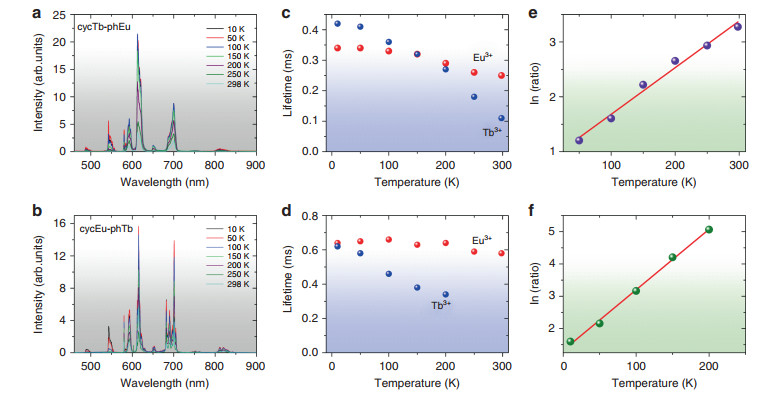
Fig. 7 Emission spectra (λexc = 355 nm) and thermometric properties of complexes.
cycTb-phEu a and cycEu-phTb b recorded at 10, 50, 100, 150, 200, 250, and 298 K. Temperature-dependent long-term lifetimes of 5D0 (Eu3+) and 5D4 (Tb3+) for cycTb-phEu (c) and cycEu-phTb d. Natural logarithm of the emission count ratio 5D0 → 7F4 (Eu3+): 5D4 → 7F5 (Tb3+) plotted against temperature for e cycTb-phEu with fitting equation y = (0.83 ± 0.10) + (0.0085 ± 0.0005)x, Radj2 = 0.9823 and f cycEu-phTb with fitting equation: y = (1.32 ± 0.07) ± (0.0188 ± 0.0006)x, Radj2 = 0.9960The different temperature-dependent luminescent emission of the 5D4 → 7F5 transition of Tb3+ and the 5D0 → 7F4 transition of Eu3+ indicates that these dinuclear complexes are potential self-referencing thermosensitive probes that do not require any cumbersome calibration of the emission intensity. This makes a luminescent thermometer more reliable. The two target emission intensity measurements lie in clear spectral windows so that background interference is absent. The ratio of the emission intensities, R, follows an exponential growth curve with temperature (Fig. S12), and the natural logarithm of the ratio is plotted against temperature in Fig. 7e, f with cycEu-phTb providing the better fit. The relative sensitivity is S = (dR/dT)/R, i.e., the change in ratio with temperature divided by the value of the ratio. Since, from Fig. S12b:
$$ {R = A\,{\rm{x}}\,{\rm{exp}}\left( {B{\rm{x}}{\it{T}}} \right){\rm{,where}}\,{{A = 3}}{\rm{.96}}\,{\rm{and}}\,{{B = 0}}{\rm{.01857K}}^{{ - 1}}} $$ (3) $$ S = {{B}} $$ (4) and the relative sensitivity is constant at 1.86%K−1. The temperature resolution, dT, is given by:
$$ {{d}}T = \left( {1/S} \right)\times \left( {{{d}}R/R} \right) $$ where dR is the error (standard deviation) in R value at temperature T and is < 1 ℃ at T > 200 K (Fig. S12c).
The dyad cycEu-phTb is therefore suitable for use as a luminescent self-referencing thermo-monitor with temperature responsive luminescence and lifetimes.
Structure
Synthesis
Singlet and triplet states
Lanthanide luminescence at low temperature
Emission decay lifetimes of Tb3+ and Eu3+ and temperature dependence
Energy transfer from Tb3+ to Eu3+
Energy transfer from antenna to Eu3+ ion
Thermometric properties
-
A strategy to synthesize hetero-dinuclear complexes that consist of chemically similar lanthanides has been introduced by which cycTb-phEu and cycEu-phTb were produced. The different energy gaps between the ligand triplet state and the acceptor lanthanide ion states, as well as the Tb3+ to Eu3+ energy transfer, result in different luminescence performances for each metal ion, giving an increased temperature-dependent luminescent emission ratio for europium over terbium. Both dinuclear complexes illustrated excellent temperature sensitivity over a wide temperature range, with cycEu-phTb having the best potential as an optical thermometer using an excitation wavelength of 355 nm. The temperature-dependent energy transfer between the two lanthanide ions that quenches Tb3+ emission has been rationalized by observing the importance of the 7F1 state at room temperature. LUMPAC calculations point to the energy transfer to Eu3+ from higher ligand states, rather than from the lowest triplet state.
-
All chemicals were purchased and received without further purification. NMR spectra were measured on a Bruker400 (400 Hz) magnetic resonance spectrometer with chemical shifts expressed as parts per million (ppm) and coupling constants, J, as Hertz (Hz). Mass spectrometry was taken on an ABI QSTAR Elite quadrupole-time-of-flight mass spectrometer using electrospray ionization (ESI) as the ion source. The HPLC measurements were conducted on an Agilent 1200 series HPLC (Column: Vision HT C18 HL 5u, length 250 mm, Serial No. 5151920 ID 4.6 mm). Fourier transform-infrared spectroscopy was performed on a PerkinElmer Paragon 1000 PC FT-IR spectrometer with KBr tablets. The emission spectra and decay lifetime measurements were recorded on a Horiba fluorescence spectrometer with a xenon lamp and a SpectreLED as the excitation sources, on an iHR550 spectrometer with a Nd3+:YAG laser as the excitation source, and on a Mini-tau from Edinburgh Instruments.
-
K.L.W. acknowledges grants from The Hong Kong Research Grants Council (HKBU 22301615) and from Hong Kong Baptist University (FRG 2/17-18/007).
-
The experimental work was performed by G.B.; the manuscript was written by G.B. and P.A.T.; calculations were performed by P.A.T.; overall supervision of the project was performed by K.-L.W. and D.J.
-
The authors declare that they have no conflict of interest.
-
Supplementary information is available for this paper at https://doi.org/10.1038/s41377-018-0097-7.






 DownLoad:
DownLoad: